Thermostability of Synthetic Proteins
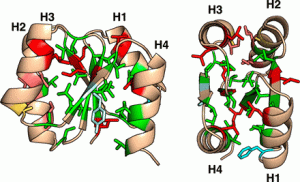
Stability of synthetic proteins
“De novo designed proteins exhibit a remarkable property of extremely high thermal stability compared with naturally occurring proteins. The designed proteins are completely optimized for folding; the backbone structures are created by using a set of rules that relate local backbone structures to preferred tertiary motifs and the side chains are designed to favor both the local backbone structures and the entire tertiary structures. Here, we (researchers at the National Institutes for Natural Sciences in Japan) found that one of the de novo designed proteins, which was mutated to fill the core with mostly valine residues, still has the folding ability and shows high stability (Tm = 106 °C) even with its reduced and loosened core packing. This result supports the importance of local backbone structures to protein folding.” MORE
Image Credit: National Institutes for Natural Sciences in Japan and PNAS.org


