Trouble Shooting Gene Therapy
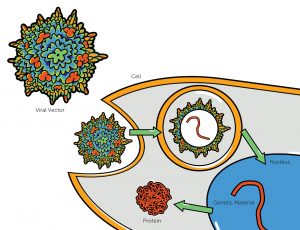
Gene therapy via viral vector
Making safe and serviceable viral vectors for gene therapy has many challenges—from empty to overfilled AAV capsids along with various contaminants. This piece describes how to overcome those problems. “A gene therapy in development must also be tested to ensure that it is free of residual, process-related impurities such as polyethylenimine, iodixanol, poloxamer, and other excipients that must be removed in the final product to ensure safety. Few research and manufacturing facilities have the equipment and expertise necessary to perform this kind of testing, and it is advisable to find one that has experience testing polymers, extractables and leachables to examine if components of the manufacturing equipment or drug’s packaging are not contaminating the final product.” MORE
Image Credit: Avomeen


