Advanced Noninvasive Blood Monitor
September 2, 2014 | Terry Sharrer
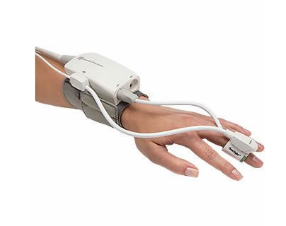
EdwardscClearSight Noninvasive Blood Monitor
Edwards Lifesciences (Irvine, CA) has received FDA approval for its “Clear Sight” blood monitor—“The inflatable finger cuff provides access to key hemodynamic parameters, including Stroke Volume (SV), Stroke Volume Variation (SVV), Cardiac Output (CO), Systemic Vascular Resistance (SVR), and Continuous Blood Pressure (cBP).” While this is designed for monitoring post-surgical patient, the device could be used for passive, continuous blood monitoring studies of hypertension. MORE
Image Credit: Edwards Lifesciences


